Categorising antibiotics by risk
There are many different organisations that have classified antibiotics by risk.
These include:
World Health Organization (WHO)
Critically Important Antimicrobials in Human Medicine
This document had a global perspective and considers:
- The importance of different antibiotics for treating bacterial infections in human medicine and the availability of alternatives. For example, is it used for treating serious life-threatening diseases (for example, meningitis/pneumonia) with limited alternatives?
- The risk of bacteria/resistance genes spreading from people to animals. For example, does it cause resistance in zoonotic or commensal bacteria that can pass between animals and people (for example, Salmonella, Campylobacter or E. coli) and does it cause resistance in transferable mobile genetic elements (for example, plasmids) which can pass from one bacterial cell to another.
On this basis antibiotics were categorised as:
- Important
- Highly Important
- Critically Important
- Highest Priority Critically Important
World Organisation For Animal Health (WOAH, formally OIE)
OIE List of antimicrobials of veterinary importance
This document considers the importance for treating bacterial infections in veterinary medicine and the availability of alternatives. It therefore reflects concerns about the development of resistance in the veterinary (rather than human) population.
On this basis, antibiotics were categorised as:
- Important,
- Highly Important
- Critically Important
However, across the UK we tend to focus on the risk categorisation produced by the Antimicrobial Advice Ad Hoc Expert Group (AMEG) as this:
- considers the risk from a European context
- considers both the importance for treating bacterial infections and the availability of alternatives in both human and veterinary medicine
- provides a comprehensive model for assessing the risk of bacteria/resistance genes spreading from people to animals
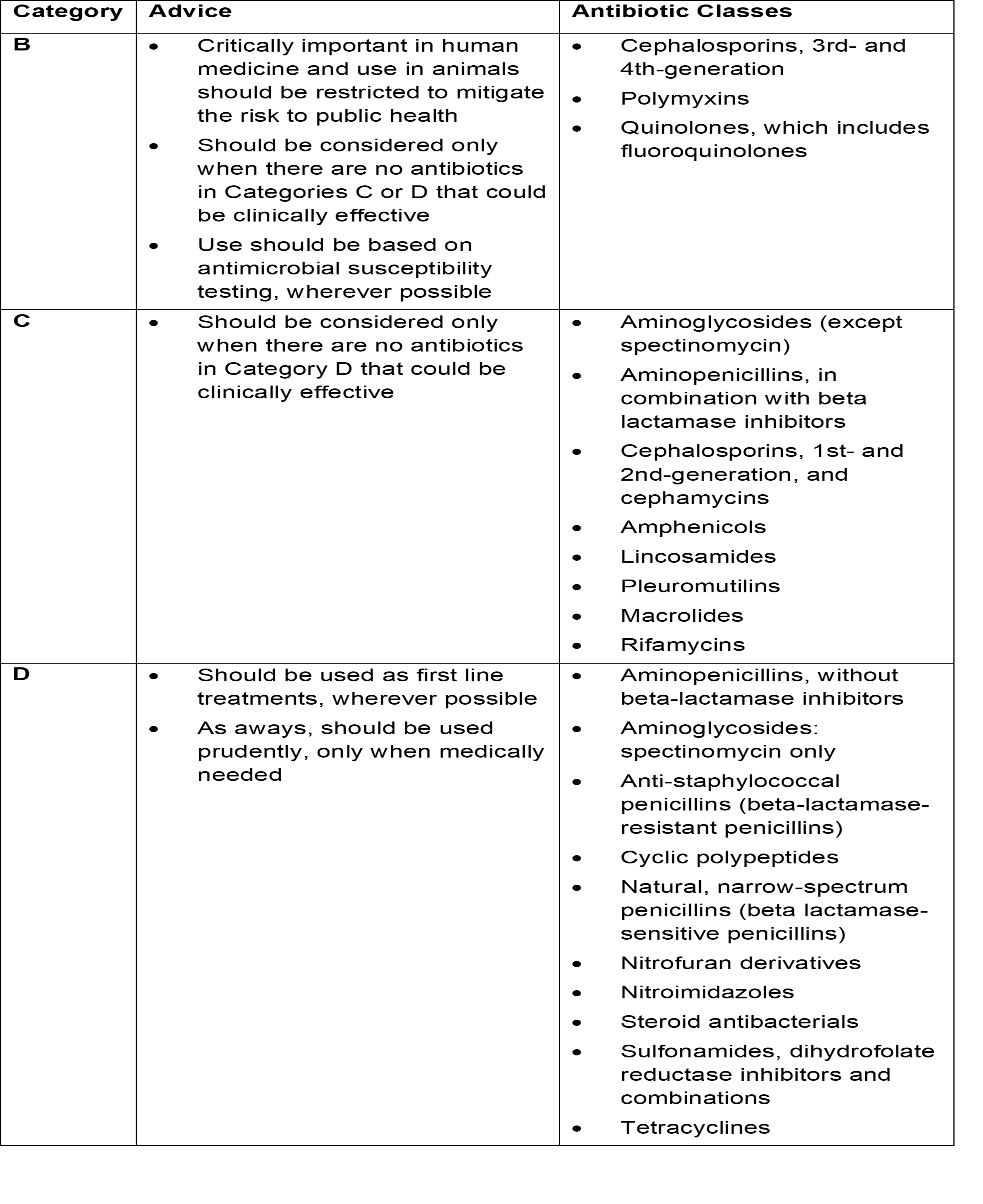
On this basis, antibiotics classes not authorised as veterinary medicines in the UK were added into category A whereas those which were authorised were categorised as follows:
Table showing category B - D of AMEG categorisations
How should the categorisation be used?
The AMEG guidelines is not a legislative document, but it can be used to:
- ensure that critically important antibiotics are included in antimicrobial susceptibility monitoring programmes
- develop prudent use/treatment guidelines
- prioritise risk management strategies
How has the categorisation been used in the UK?
In meat poultry:
- Under rules of the industry body British Poultry Council and the farm assurance scheme Red Tractor, the use of 3rd and 4th generation cephalosporins and colistin is not permitted, whereas fluoroquinolones can only be used as a last resort under veterinary direction, supported by a veterinary statement outlining the justification for use and a written agreement from a director of the company purchasing the birds.
In laying hens:
- Under the British Lion Code, the use of fluoroquinolones in day old chicks and the use of 3rd and 4th generation cephalosporins is prohibited.
In cattle and sheep:
- The farm assurance scheme Quality Meat Scotland (QMS) requires that any use category B antibiotics must be as a last resort, and supported by a veterinary statement outline justification for use.
- The farm assurance scheme Red Tractor also requires that any use of a category B antibiotic is supported by a veterinary statement outlining the justification for use, including sensitivity testing and/or diagnostics (this can occur in parallel with treatment)
- Red Tractor Beef and Lamb Standards
- Red Tractor Dairy Standards
- Example of a Red Tractor justification sheet for use of category B antibiotics in dairy
In pigs:
- The farm assurance scheme Quality Meat Scotland (QMS) requires that HP-CIAs are only used when there is no suitable alternative, or where sensitivity tests have been completed beforehand, to prove their efficacy and need to treat the specified target condition. It also specifies that their use must be justified in the VHWP and an antibiotic reduction plan must be in place to prevent recurrent use.
- Under the farm assurance scheme Red Tractor, HP-CIAs must only be used as a last resort and supported by a veterinary statement outlining the justification for use, including sensitivity and/or diagnostic testing (this can occur parallel with treatment).
- The industry body the Pig Veterinary Society also produce industry prescribing principles, which closely mirror the AMEG categorisation. Under Red Tractor, vets must prescribe in accordance with these principles.
In dogs and cats:
- The BSAVA/ SAMSoc PROTECT Me poster states that category B antibiotics can only be used when first line antibacterials are inappropriate or ineffective. If urgent treatment required, samples for culture and sensitivity should be submitted before starting these agents, and therapy adapted.
In rabbits:
- The BSAVA/ SAMSoc PROTECT Me rabbit poster says that category B antibiotics should only be used when there is evidence that other veterinary authorised products are unlikely to be effective (e.g. culture and sensitivity results).
In horses:
- The BEVA PROTECT Me Toolkit encourages practices to have their own policies highlighting which antibiotics are first-line (green), alternatives (blue) and protected (orange). In the example given, category B antibiotics are marked as protected.